Dissecting protein assemblies
New molecular details from mitochondria
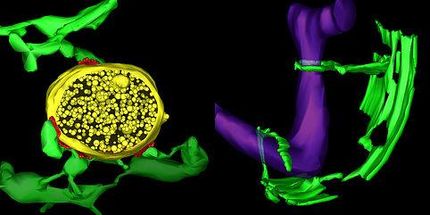
Super-resolution MINFLUX nanoscopy, developed by Nobel laureate Stefan Hell and his team, is able to discern fluorescent molecules that are only a few nanometers apart. In an initial application of this technique to cell biology, researchers led by Stefan Hell and Stefan Jakobs have now optically dissected the distribution of individual proteins in an about 20-nanometer-sized protein cluster within a cellular organelle in 3D using multiple colors. MINFLUX nanoscopy thus proves to be an extremely powerful tool to find out if and how proteins group inside the cell, at the length scale of the proteins themselves.
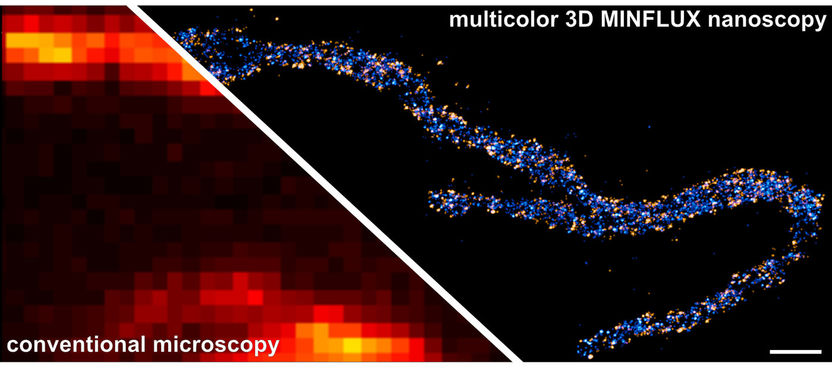
Multi-color 3D MINFLUX nanoscopy visualizes the cellular distribution and relative position of proteins that are only a few nanometers apart. The picture shows a mitochondrion from a human skin cell in which two proteins in the inner mitochondrial membrane are stained: A subunit of the MICOS complex (Mic60), is colored in orange, a subunit of the mitochondrial ATP synthase (ATPB), is colored in blue. The scale bar has a length of 500 nanometers.
© Till Stephan & Jasmin Pape / Max Planck Institute for Biophysical Chemistry
With the MINFLUX concept, Stefan Hell cleverly combined the strengths of the two highest-resolution fluorescence nanoscopy techniques available to date: PALM/STORM and the STED method that Hell developed and for which he received the Nobel Prize in Chemistry in 2014. Introduced in 2016, MINFLUX uniquely provides a resolution of up to only a few nanometers inside cells. Furthermore, MINFLUX can track molecules moving in the cell up to one hundred times faster than conventional microscopy methods. Since developing the method, his team has optimized this approach for biological applications.
“In recent years, my colleagues Klaus Gwosch, Francisco Balzarotti, and I worked hard on making fluorescent molecules in cells visible with maximum molecular resolution in two colors and 3D using MINFLUX. We recently succeeded,” says Jasmin Pape, a researcher in Stefan Hell's Department of NanoBiophotonics. “Since biologists often analyze complex samples, we have adapted the method to meet this requirement. We can now image and even quantitatively examine intricate structures and protein clusters in cell organelles.”
A key technology in cell biology
MINFLUX has successfully mastered the practical test. “With this unique technique, we have achieved a 3D-resolution of only a few nanometers in the powerhouses of a cell, the mitochondria,” reports Jakobs, research group leader at the Max Planck Institute (MPI) for Biophysical Chemistry and professor at the Department of Neurology at the University Medical Center Göttingen (UMG). “This is the highest resolution of sub-cellular protein clusters ever achieved in 3D. I am very pleased about the first biological application of our method,” says Hell, Director at the MPI for Biophysical Chemistry and the MPI for Medical Research. “The excellent cooperation at our MPI, which seamlessly combines biology, chemistry and physics under one roof, has made this success possible. For cell biology, MINFLUX will be a key technology in the coming years, because it will enable scientists to map out subcellular organelles and protein clusters on a molecular level.”
The optical resolution of MINFLUX nanoscopy is so good that the researchers have to keep an eye out for any imperfections in sample preparation and labeling. “In fact, sample preparation and labeling have become the limiting factor – it is no longer the microscope’s optical resolution," explains Till Stephan, researcher in Jakobs’ group. “The decisive requirements for a high-quality image are the retention of the structure in the sample and the distance between the fluorescent markers and the proteins of interest.”
New molecular details from mitochondria
Jakobs and his co-workers have been researching the structure and function of mitochondria for many years. The molecular powerhouses supply the energy necessary to keep the metabolism of our body’s cells going. As a result, they have an exceptional structure comprising of a smooth outer membrane and an inner membrane folded in the shape of fingers. The protein machinery on these finger-like cristae supplies the human body with about 75 kilograms of the energy storage molecule called ATP every day. At the crista junctions, the locations where cristae meet the inner boundary membrane, an important molecular machine called MICOS is at work. MICOS consists of several proteins that work together to bend the mitochondria’s inner membrane into shape.
By harnessing the MINFLUX nanoscope, Jakobs and his team have resolved surprising details about the crista junctions. “Our results suggest that the MICOS proteins are very heterogeneously distributed around the crista junctions,” states the biologist. His group aims to build on this impressive work, using MINFLUX to investigate how the interaction of the key MICOS proteins is controlled. Since many diseases of the nervous system and the muscular system are associated with defective mitochondrial architecture, future findings might contribute to a better understanding of how such conditions develop.
Commercial MINFLUX nanoscope
In the future, Jakobs and his team will begin using a brand new commercial MINFLUX nanoscope: one of only four instruments worldwide, which is set to be installed in Göttingen within the coming month. The German Research Foundation (DFG) will fund the project led by Jakobs with 2.3 million euros. The new high-performance nanoscope will be part of the equipment in the biologist's laboratory at the UMG, and fellow researchers at the Göttingen Campus will also be able to use it. “The spectrum of applications using MINFLUX ranges from biomolecular chemistry to cardiology, audiology, and neurology and will further strengthen Göttingen as a haven for interdisciplinary biomedical research,” Jakobs emphasizes.