The heaviest element ever chemically studied
Experiments succeed in determining properties of moscovium and nihonium
An international team led by scientists of GSI/FAIR in Darmstadt, Johannes Gutenberg University Mainz and the Helmholtz Institute Mainz, succeeded in determining the chemical properties of the artificially produced superheavy elements Moscovium and Nihonium (elements 115 and 113). Moscovium thus becomes the heaviest element ever chemically studied. Both of the newly characterized elements are more chemically reactive than flerovium (element 114), which was previously studied at GSI/FAIR. The results are published in the journal Frontiers in Chemistry.
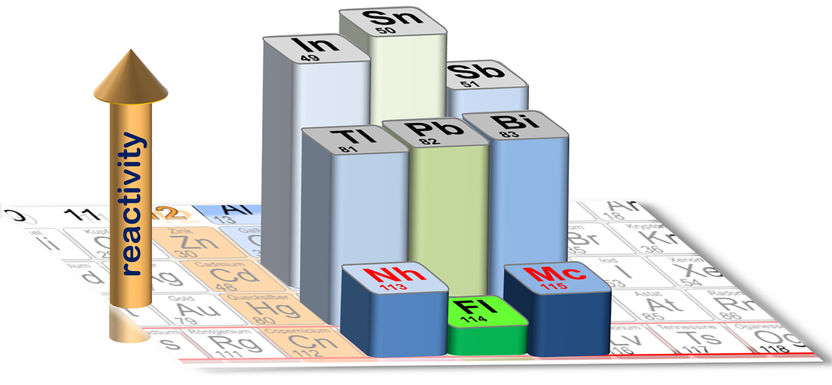
Cutout of the periodic table: The height of the bars of the highlighted elements represents the strength of the bonding on a quartz surface.
A. Yakushev/Ch.E. Düllmann
With this result, experiments at GSI/FAIR now provide data on the three superheavy elements 113, 114 and 115, allowing for a reliable classification of their properties and an assessment of the structure of the periodic table in this extreme region. As elements become heavier, their many protons in the nucleus accelerate the electrons spinning around the core to ever higher velocities – so high that effects explicable only with Einstein’s famous relativity theory kick in. The sheer speed renders electrons heavier.
In lead (element 82), for example, the effects of such processes are already at work and contribute to the chemical processes in lead batteries. The neighbors left and right – thallium and bismuth – behave differently. The effect, albeit small, is localized at lead. Could a superheavy element be a lead alternative? How about the heavier neighbor down the group of the periodic table, flerovium, element 114, discovered and chemically studied only in the last 20 years? It was found to be quite unlike lead, transforms into a gas easily and is less chemically reactive.
To find answers, the two neighbors, elements 113, nihonium, and 115, moscoivum, needed to be tested as well. While first hints at the chemistry of nihonium were reported, nobody had so far achieved a study of the chemistry of moscovium – where the best-suited isotope exists for only about 20 hundredths of a second.
This very feat has now been achieved by the international collaboration at GSI Helmholtzzentrum für Schwerionenforschung in Darmstadt, Germany. The team reported that both neighbors, nihonium as well as moscovium, show a higher chemical reactivity than the intermediate flerovium. The local effect seen in lead is thus also seen in flerovium, however, much more strongly, which comes as no surprise given the much higher nuclear charge.
The observation of a mere handful of atoms was sufficient for obtaining this result. Still, it took two months of continuous around the clock work at the GSI/FAIR’s heavy ion accelerator facility to achieve this. To produce the superheavy elements, the team irradiated thin foils containing americium-243 (element 95), itself an artificial element, with intense ion beams of calcium-48 (element 20). Their fusion led to nuclei of moscovium-288 (element 115), which transformed within a fraction of a second to nihonium-284 (element 113).
Inert gas flushed both elements through a detector array covered with a thin quartz layer. The detectors register the decay of the individual superheavy atoms and determine if the atoms form a chemical bond with quartz strong enough to hold them where they first encounter the surface. Weaker binding leads to further transport by the gas. In this way, the pattern registered in the detector array provides information on the strength of the chemical bonds – hence the chemical reactivity of the elements. Elements of low reactivity could even exit the array, but only to encounter gold-covered detectors. Bonds with gold are generally stronger than with quartz, thus ensuring that each studied atom is indeed retained and registered.
“Thanks to a newly developed setup for chemical separation and detection in combination with the electromagnetic separator TASCA, our gas chromatography studies could be extended to more reactive chemical elements like nihonium and moscovium,” explains Dr. Alexander Yakushev of GSI/FAIR, the spokesperson of the international collaboration. “We have succeeded in increasing the efficiency and reducing the time required for the chemical separation to such an extent that we were able to observe the very short-lived moscovium-288, and at an even larger rate of about two detected atoms every week its daughter nihonium-284.”
In total, four moscovium atoms were registered, all in the quartz-covered array. Among the 14 detected nihonium atoms, deposition mostly on quartz was observed, pointing to the formation of a chemical bond. One atom reached the gold-covered array, indicating that the quartz bond is not very strong. This is in contrast to the behavior of the lighter homologs thallium (for nihonium) and bismuth (for moscovium), which are both known to form strong bonds with quartz. Similarly, lead, the homolog of flerovium, forms strong bonds with quartz, whereas flerovium does not.
The complete data set on these elements shows that the superheavy elements are much less reactive than their lighter homologs, ascribed to inertness associated with the occurrence of relativistic effects. The most pronounced effect is seen locally at flerovium, which is still a metal, but a very weakly reacting one – a behavior that indicates the presence of closed electron (sub)shells, almost as in the non-reactive noble gases. The results demonstrate the influence of Einstein’s relativity theory in the periodic table and at the same time set a new record for the heaviest element ever chemically studied.
With technological advances, new requirements for materials emerge. Could new elements contribute? Like cars transform from fossil to electricity-driven, also other items of our daily life phase out, being replaced by technology based on novel materials. The first flerovium-based device is not around the corner yet. Only single atoms per week – which last for less than a second – can currently be produced. As technology advances, this may change, eventually making larger amounts available. Whether they might serve in future batteries, as medical agents, or enrich our lives in ways inconceivable today, we do not know. But thanks to the groundbreaking experiments at Darmstadt, future researchers will have a head-start and already know the chemical character of these new materials. The result also opens new perspectives for the international facility FAIR (Facility for Antiproton and Ion Research), which is currently under construction in Darmstadt.
Original publication
A. Yakushev, J. Khuyagbaatar, Ch. E. Düllmann, M. Block, R. A. Cantemir, D. M. Cox, D. Dietzel, F. Giacoppo, Y. Hrabar, M. Iliaš, E. Jäger, J. Krier, ... L. G. Sarmiento, B. Schausten, U. Scherer, P. Thörle-Pospiech, N. Trautmann, M. Wegrzecki, P. Wieczorek; "Manifestation of relativistic effects in the chemical properties of nihonium and moscovium revealed by gas chromatography studies"; Frontiers in Chemistry, Volume 12, 2024-9-23