Solved protein structure holds key to much-needed therapies for metabolic disorder
phenylketonuria, also known as PKU, is the most common inherited disease affecting amino acid metabolism. Children are tested for PKU at birth, and babies diagnosed with the disease must adhere to a highly restricted diet to prevent permanent intellectual impairment. Recent advances reveal a strong need for new therapies to combat cognitive dysfunction and psychiatric disorders that arise due to dietary lapses throughout life.
In a study published in Proceedings of the National Academy of Sciences, Fox Chase Cancer Center -- Temple Health researchers made tremendous strides toward that goal by shedding new light on the structure of phenylalanine hydroxylase (PAH) -- the enzyme that is defective in PKU patients. Researchers from Drexel University College of Medicine, the Perelman School of Medicine at the University of Pennsylvania and the University of Canterbury in New Zealand contributed to the study.
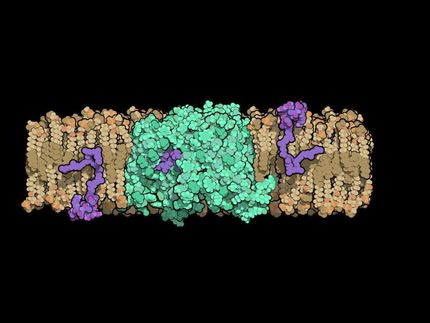
"We have solved the first X-ray crystal structure of full-length PAH, which has defied crystal structure determination for decades," said senior study author Eileen K. Jaffe, professor of Molecular Therapeutics at Fox Chase. "This structure will help us understand the molecular origins of PKU and is an important advance in developing much-needed drugs for patients."
PKU is an inherited disorder characterized by low levels of functional PAH, which converts an amino acid called phenylalanine to other important compounds in the body. Defects in PAH cause phenylalanine to build up to toxic levels in the body. Because nerve cells in the brain are particularly sensitive to phenylalanine levels, excessive amounts of this substance can cause defects in brain development or abnormal brain function.
If patients do not strictly follow a low-phenylalanine diet for their entire lives, they risk severe consequences, including seizures, delayed development, behavioral problems, psychiatric disorders, and permanent intellectual disability. Maintaining this strict diet can be a challenge for people living with PKU -- a problem made more difficult by limited development of non-dietary therapies based on knowledge of the structure of PAH.
In the new study, Jaffe and her team overcame substantial technical hurdles to solve the first X-ray crystal structure of full-length PAH. This structure consists of an asymmetric arrangement of four identical subunits in a low-activity form that predominates at low levels of phenylalanine. Additional solution studies show that a distinct four-unit structure, known as a tetramer, characterizes the activated form of PAH. The evidence that alternate forms of PAH are represented by distinct tetramer structures supports Jaffe's recently proposed model for the activation of PAH by dietary phenylalanine.
"This study provides the first complete view of the enzyme in a tetrameric form, which was not possible with prior partial crystal structures," Jaffe said. "It also facilitates the interpretation of a wealth of biochemical and structural data that was previously impossible to evaluate."
Using what they have learned from this work, Jaffe and her team are now working on solving the structure of activated PAH, which functions to prevent blood phenylalanine from rising to neurotoxic levels. "Knowledge of both PAH structures will help us use structure-based drug design techniques to develop drugs that will favor activated PAH as a therapeutic approach," Jaffe said.
In the end, this research could pave the way for new treatment strategies not only for PKU, but also for cancer and other diseases. "Both inborn errors of metabolism and cancer occur when our proteins do not function as they should," Jaffe said. "Our focus on how different protein assemblies can be harnessed to control protein function has tremendous potential for drug discovery. However, this approach requires that protein chemists and pharmaceutical companies embrace an expanded view of how small molecule therapeutics can work."
Most read news
Topics
Organizations
Other news from the department science

Get the analytics and lab tech industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.