Bacterial organizational complexities revealed
For the first time, scientists have visualized the fine details of bacterial microcompartment shells - the organisms' submicroscopic nanoreactors, which are comprised completely of protein.
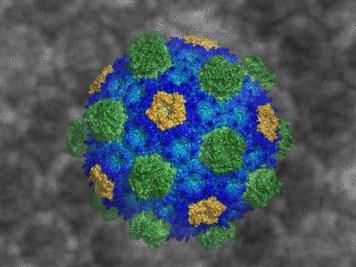
Michigan State University scientist provides first detailed snapshot of bacteria's building blocks. Revealing this near-universal structure could lead to new medicines and bio-engineered bacteria for fuels, new fertilizers and more.
Courtesy of MSU
The results show how the architectural principles of bacterial microcompartments, or BMCs, apply to both "good" and "bad" bacteria that use these nanoreactors to provide energy for infections. The findings open the door to identifying vulnerable targets to combat pathogenic bacteria as well as to bioengineer new kinds of designer nanoreactors in beneficial bacteria to enhance their performance.
"We've produced a detailed snapshot - at atomic-level resolution - of the membrane of bacterial organelles," said Cheryl Kerfeld, the Hannah Distinguished Professor of Structural Bioengineering in the MSU-DOE Plant Research Lab and co-lead author. "By seeing the intact bacterial organelle shell, we now understand how the basic building blocks are put together to construct the organelle membrane."
In human and animal cells, organelles are lipid-based. In contrast, these BMCs are composed of hundreds of copies of several types of proteins - hexamers, pentamers and trimers.
"What allows things through a membrane is pores," said Markus Sutter, MSU senior research associate, Berkeley Lab affiliate scientist and co-lead author. "For lipid-based membranes, there are membrane proteins that get molecules across. For BMCs, the shell is already made of proteins, so the shell proteins of BMCs not only have a structural role, they are also responsible for selective substrate transfer across the protein membrane."
The structure's appearance is reminiscent of buckyballs, a class of molecules that resemble Buckminster Fuller's geodesic domes, whose discovery earned a Nobel Prize in chemistry.
"Our results provide the structural basis to design experiments to explain how molecules cross the organelle shell, how specific enzymes are targeted to the inside and how the shells self-assemble," said Kerfeld, who's also an affiliate of Lawrence Berkeley National Laboratory. "This work also provides the foundation for the development of therapeutics to disrupt the assembly and function of the BMCs found in pathogens or enhance those that play a role in CO2 fixation."
The structures are comparatively large - 6.5 megadaltons and can contain approximately 300 average sized proteins. In comparison, one megadalton is comparable to the mass of 1 million hydrogen atoms. Viewing the structures required patience in coaxing the BMCs to form crystals and a large quantity of computational power due to the number of atoms involved.
To image the BMCs, Kerfeld's team used Berkeley Lab's powerful Advanced Light Source and SLAC National Accelerator Laboratory, both of which utilize X-rays to visualize crystallized proteins.
It took the team about two years to finally see this structure, and there were challenges at every step of the way because the shell is so large and unusual. The structure described is likely to become the textbook model of the membrane of primitive bacterial organelles, Kerfeld said.
"I can remember the first time I saw images of animal cell organelles, taken with an electron microscope, when I was taking freshman biology in college; seeing those inspired me and helped shape my career path," she said. "I remembered that excitement when I became one of the first people to see this structure."
Original publication
Sutter, Markus and Greber, Basil and Aussignargues, Clement and Kerfeld, Cheryl A.; "Assembly principles and structure of a 6.5-MDa bacterial microcompartment shell"; Science; 2017